Healthcare-Acquired Infections
Reduce Patient Risk of Transfusion Transmitted Infectionthe Healthcare-associated infections (HAIs), also known as Hospital Acquired Infections, create a major strain on our healthcare system today
in terms of both patient safety and the financial stability of healthcare providers.
The elimination of HAI’s is a priority of the Department of Health and Human Services.1 Hospitals limit their reporting of HAI’s to device-associated infections, selected surgical-site infections, and infections due to Clostridium difficile and methicillin-resistant Staphylococcus aureus (MRSA).2 There has been some success reported in the prevention for some infections such as central line associated bloodstream infections or CLABSI.3
The CDC’s National Healthcare Safety Network (NHSN) cannot provide national-scale data on the overall burden and distribution of HAI’s across acute care patient populations. A multistate prevalence survey of HAI’s and use of antimicrobial agents was done between 2009 and 2011 in a large-scale survey to estimate the prevalence of HAI in acute care hospitals to determine the distribution of these infections according to infection site and pathogen. The HAI Prevalence Study was published in the New England Journal of Medicine in 2014. This study generated estimates of the national burden of these infections. There were 11, 290 patients included in the survey between May and September 2011. The median patient age was 58 years. 51.9% of the patients were receiving, or were scheduled to receive, antimicrobial agents at time of the survey.4 There were 481 pathogens reported for 372 of 504 HAI’s. The breakdown of these infections are shown in the table below.
Distribution of 504 Health Care-Associated Infections
| Rank | No. of Infections | Percentage of all Health care Associated Infections (95% CI) | |
| Pneumonia † | 1 (tie) | 110 | 21.8 (18.4-25.6) |
| Surgical-site infection | 1 (tie) | 110 | 21.8 (18.4-25.6) |
| Gastrointestinal infection | 3 | 86 | 17 (14.0-20.5) |
| Urinary tract infection ‡ | 4 | 65 | 12.9 (10.2-16.0) |
| Primary Bloodstream infection § | 5 | 50 | 9.9 (7.5-12.8) |
| Eye, ear, nose, throat, or mouth infection | 6 | 28 | 5.6 (3.8-7.8) |
| Lower respiratory tract infection | 7 | 20 | 4.0 (2.5-6.0) |
| Skin and soft-tissue infection | 8 | 16 | 3.2 (1.9-5.0) |
| Cardiovascular system infection | 9 | 6 | 1.2 (0.5-2.5) |
| Bone and joint infection | 10 | 5 | 1.0 (0.4-2.2) |
| Central nervous system infection | 11 | 4 | 0.8 (1.3-1.9) |
| Reproductive tract infection | 12 | 3 | 0.6 (0.2-1.6) |
| Systemic infection | 13 | 1 | 0.2 (0.01-1.0) |
The advent of the Affordable Care Act highlighted the costs of healthcare-associated infections (HAIs) and created new incentives to reduce rates of these events. The CDC developed a scoring system based on catheter, central line, surgical site and bacteremia rates to evaluate the success of hospitals at mitigating HAI rate. Although all these factors contribute to the hospital score, the factor with the greatest increase in reported instances from 2014 to 2015 was central line associated blood stream infections (CLABSI), with a predicted infection number of over 45,000 in 2015. CLABSI’s are classified as a delayed complication of Central Venous Catheters, and can lead to sepsis, shock, and even death in up to 25% of these cases. These infections may be caused by biofilm-producing bacteria such as Staphylococcus aureus and Staphylococcus epidermidis. Sources of these infections include skin flora, contamination from infused substances, or from a hematogeneous spread from an unrelated site.5
The CDC estimates additional cost per CLABSI infection to be approximately $16,550.5 Hospitals are at a significant financial risk for reduced reimbursement of costs incurred for the treatment of an HAI. https://khn.org/news/medicare-trims-payments-to-800-hospitals-citing-patient-safety-incidents/
While there are multiple sources for CLABSI, an under-recognized source of HAIs is transfusion-transmitted infection (TTI), which may be due to the transfusion of bacterially contaminated platelets.
In a study done by Erbay et al. at the University of Utah, the patient group that received blood products was found to have a higher incidence of recurrent bloodstream infections.6
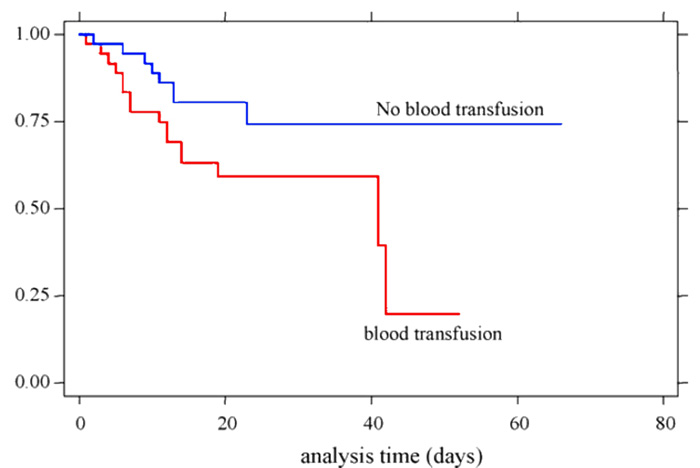
While traditional testing measures have greatly improved blood safety, particularly with regard to TTIs such as HIV, Hepatitis B & C, and West Nile virus (WNV), these approaches are reactive. Gaps in testing development and availability persist for emerging pathogens as highlighted by recent outbreaks of chikungunya, dengue, and Zika viruses. Even with costly polymerase chain reaction (PCR) testing and nucleic acid testing (NAT) methods, viral infections like WNV are still occasionally transmitted by transfusion.12,13 Testing is limited to pathogens that are known, and for which screening tests are developed over time, therefore, it is inherently limited as a reactive approach.
Cecil Aubron and colleagues published a study in Critical Care Medicine in 2017. This study included 18,000 patients in two different hospital ICU’s receiving 2,250 platelets over a 5 year period. The analysis showed that after adjustment for cofounders, including patient severity, and other blood components, platelet transfusion was independently associated with ICU acquired infection.14
CDC Report:
Fatal Sepsis Associated with Bacterial Contamination of Conventional Platelets
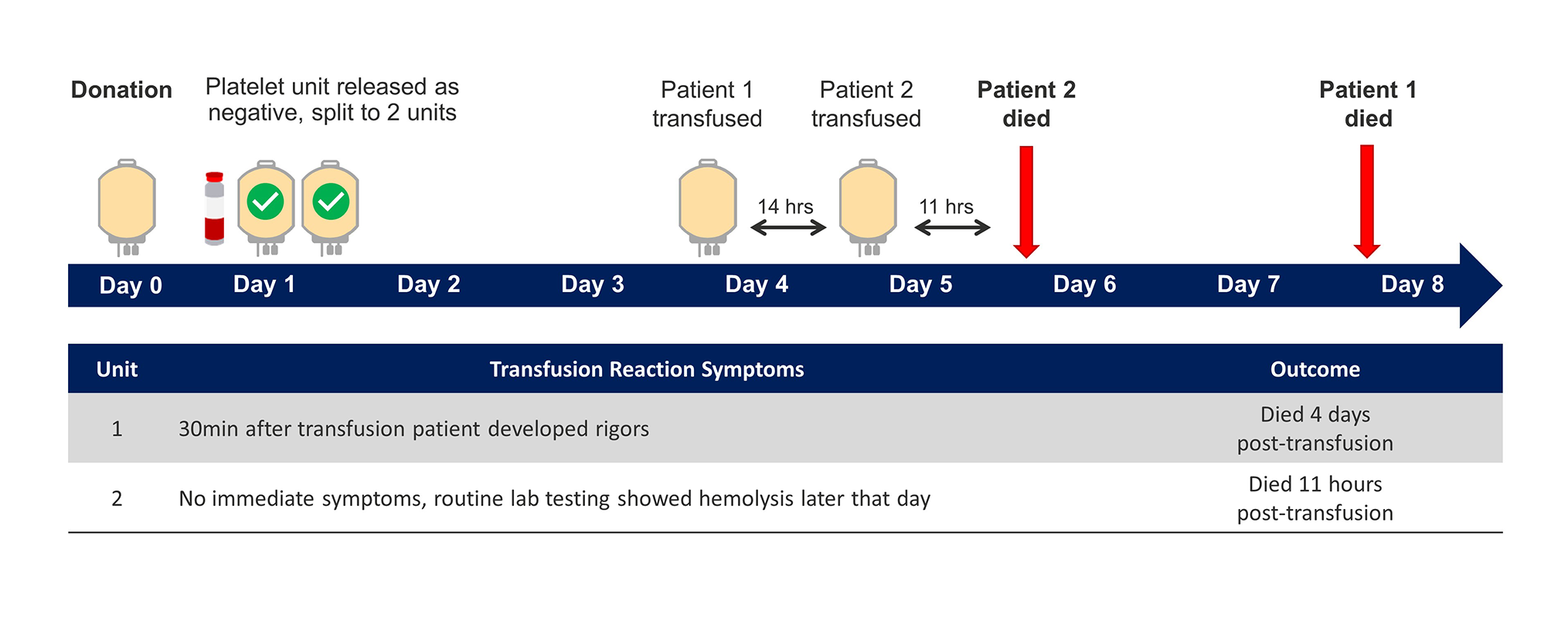
The transfusion of bacterially contaminated platelet components has been reported in recent Morbidity and Mortality Weekly Reports.15 This report published in June of 2018 describes the transfusion of platelets for five patients located in Utah and California, four of whom died as a result of these contaminated transfusions. It is important to note that all of these platelet components were tested as negative to date when they were received at the hospitals.
The first case involves one donation that was contaminated with C. perfringens, again tested negative to date when it was released from the blood center. Each of these patients received a unit of platelets from the same asymptomatic platelet donor and each patient died as a result of their platelet transfusion.15
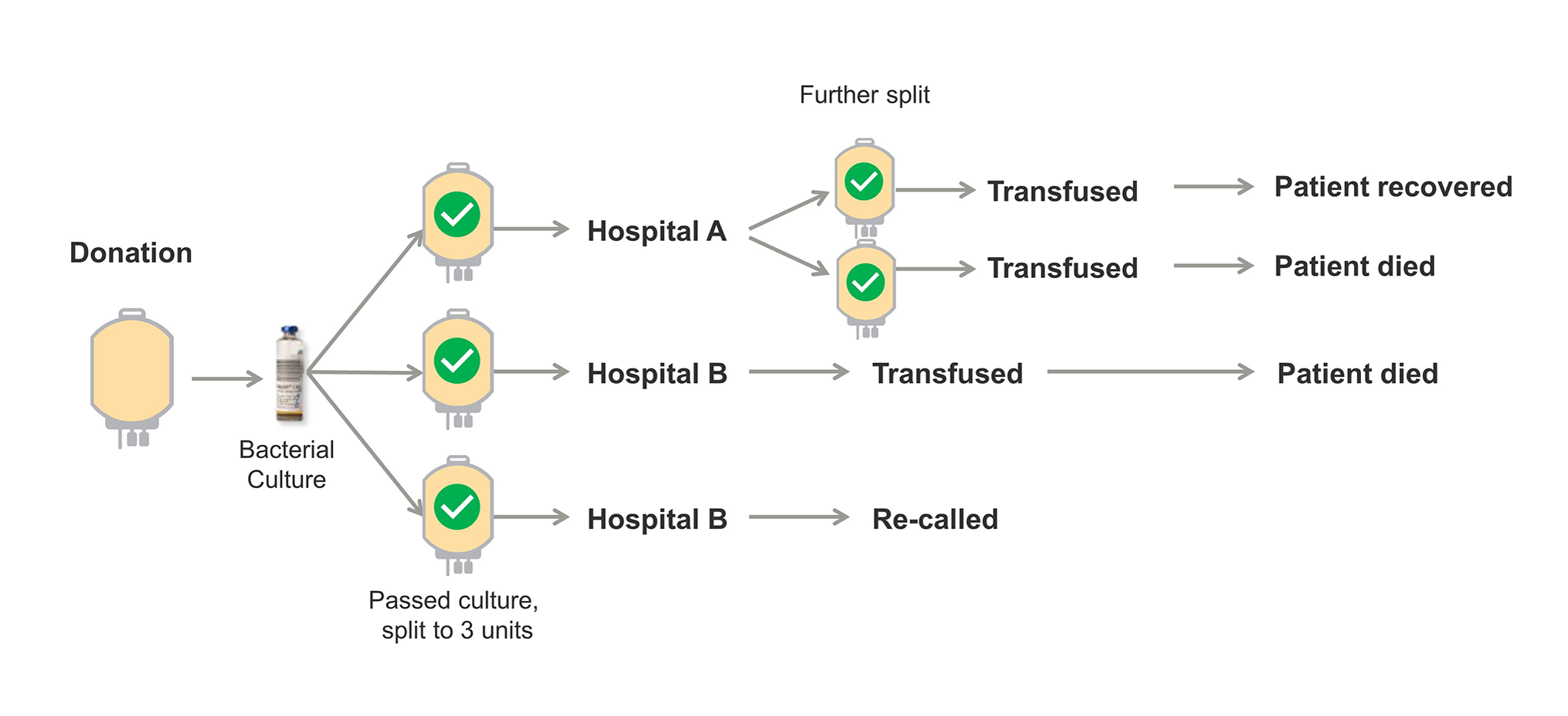
The second case involves multiple TTI’s, two of them fatal, from a single platelet unit that was contaminated with K. pneumoniae. Platelets collected from this donation was split into three separate units. One of those units was further divided into two pediatric platelet doses. Both pediatric patients that received these platelets developed symptoms consistent with a septic transfusion reaction, one of which was fatal.15
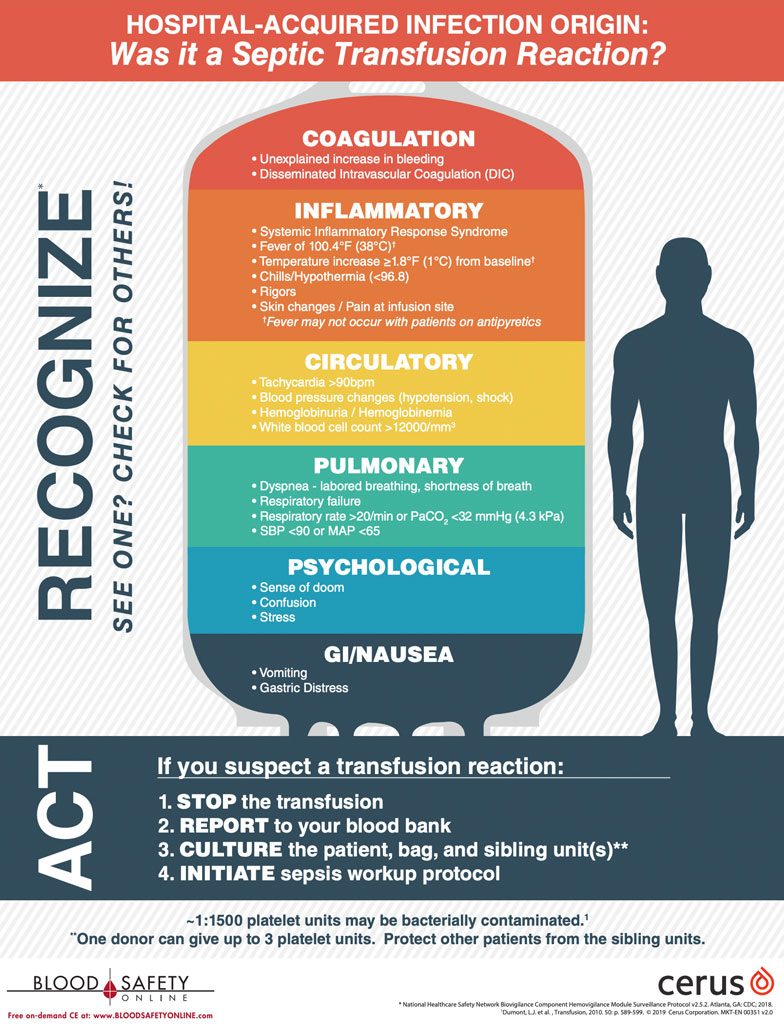
TTI’s are a potential source of HAI’s and often are not recognized as such.
The FDA estimates a 22-40% sensitivity for bacterial cultures.16 Automated bacterial culture systems may fail to detect organisms due to small sample sizes, or not completing both aerobic and anerobic cultures. Automated cultures may miss biofilm-producing organisms.17
Mitigation of HAI’s
Safer choices are now available for platelet transfusions. The INTERCEPT Blood System is an FDA-approved safety measure for use with platelets and plasma. Hospital clinicians should be vigilant in watching for HAI’s particularly one that develops following a platelet transfusion. Notifying the Blood Bank of any suspect event can prevent infections in other patients. Two-thirds of platelet units have a sibling unit or two.
The downloadable poster (11″x17″ PDF) shown below is an excellent educational resource for patient care units.
1. The Infection Prevention and HAI Portal. The Joint Commission 2018 [cited 2018 April 9]; Available from: https://www.jointcommission.org/hai.aspx. 2. CDC. Types of Healthcare-associated Infections. Centers for Disease Control and Prevention 2014 [cited 2018 April 9]; Available from: https://www.cdc.gov/hai/infectiontypes.html. 3. CDC. Healthcare-associated Infections in the United States, 2006-2016: A Story of Progress. Centers for Disease Control and Prevention 2018 [cited 2018 April 5]; Available from: https://www.cdc.gov/hai/surveillance/index.html. 4. Magill , S.S., et al., Multistate Point-Prevalence Survey of Health Care–Associated Infections. New England Journal of Medicine, 2014. 370(13): p. 1198-1208. 5. Central Line Complications; Craig Kornbau; Int J Crit Illn Inj Sci. 2015 Jul-Sep;; 5(3): 170-178. 6. Erbay, A., et al., Recurrent catheter-related bloodstream infections: Risk factors and outcome. Int J Infect Dis, 2006. 10(5): p. 396-400. 7. Inaba, K., et al., Impact of the duration of platelet storage in critically ill trauma patients. J Trauma, 2011. 71(6): p. 1766-73. 8. Aubron, C., et al., Is platelet transfusion associated with hospital-acquired infections in critically ill patients? Critical Care 2017. 21(1): p. 2. 9. Hong, H., et al., Detection of septic transfusion reactions to platelet transfusions by active and passive surveillance. Blood, 2016. 127(4): p. 496-502. 10. Ellingson, K.D., et al., Continued decline in blood collection and transfusion in the United States-2015. Transfusion, 2017. 57 Suppl 2: p. 1588-1598. 11. Kleinman, S., Reed, W. & Stassinopoulos, A. A patient-oriented risk-benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States. Transfusion. 2013. 53:1603-1618. 12. Groves, J., et al., A probable case of West Nile virus transfusion transmission. Transfusion, 2017. 57(3pt2). 13. CDC, Fatal West Nile Virus Infection After Probable Transfusion-Associated Transmission- Colorado 2012. Morbidity and Mortality Weekly Report (MMWR), 2013. 62(13): p. 622-624. 14. Aubron, C., et al., Is platelet transfusion associated with hospital-acquired infections in critically ill patients? Critical Care 2017. 21(1): p. 2. 15. Horth, R.Z., et al., Fatal Sepsis Associated with Bacterial Contamination of Platelets – Utah and California, August 2017. MMWR Morb Mortal Wkly Rep, 2018. 67(25): p. 718-722. 16. Blood Products Advisory Committee, Considerations for Options to Further Reduce the Risk of Bacterial Contamination in Platelets in 104th Meeting of Blood Products Advisory Committee 2012, BPAC: Rockville, MD. 17. Greco-Stewart, V.S., et al., Serratia marcescens strains implicated in adverse transfusion reactions form biofilms in platelet concentrates and demonstrate reduced detection by automated culture. Vox Sang, 2012. 102.