INTERCEPT PLATELETS
Protects Patients
Protect Patients* with Proven Safety and Efficacy
Help protect your patients with reduced transfusion-transmitted infection (TTI) and transfusion-associated graft-versus-host disease (TA-GVHD) risks while providing therapeutically effective platelets.1 The safety and efficacy of platelets treated with the INTERCEPT® Blood System Pathogen Reduction System (INTERCEPT Platelets) are supported by hemovigilance (HV) programs and clinical trials.
Demonstrated prevention of septic transfusion reactions in routine use
HV programs provide a comprehensive view of transfusions and potential adverse events via the surveillance of blood donations in routine use settings. Over 2.4 million INTERCEPT Platelet units have been transfused in French, Swiss, and Belgium national HV programs, with significantly reduced TTIs and no fatalities attributed to INTERCEPT Platelets.2-7
†Belgium 2018: Staphylococcus hominis was reported in a patient who recovered. This case was not thoroughly investigated for modes of contamination via storage container leaks or other environmental sources.8 ‡France 2021: Bacillus cereus transmission was reported in a patient who recovered. B. cereus is a spore-forming bacteria; the spore form is resistant to INTERCEPT.9
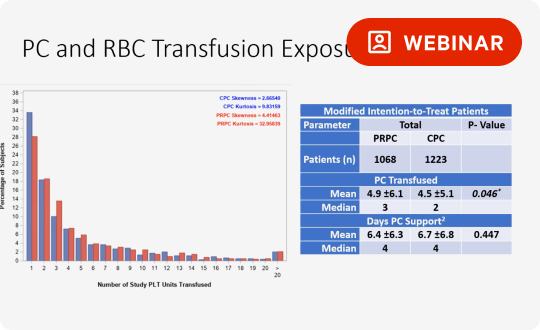
Proven therapeutic efficacy through numerous clinical trials
The INTERCEPT Blood System has been evaluated in clinical trials in which over 2000 patients received INTERCEPT Platelets. Primary endpoints were met, including bleeding criteria, a measure of hemostatic efficacy.
*There is no pathogen reduction process that has been shown to eliminate all pathogens. Certain non-enveloped viruses (e.g., HAV, HEV, B19, and poliovirus) and Bacillus cereus spores have demonstrated resistance to the INTERCEPT process. For a full list of pathogens, see Package Insert.
References:
- The INTERCEPT Blood System for Platelets Package Insert, Cerus Corporation.
- Cazenave, JP, et al. Pathogen Inactivation of Platelets. AABB Press: Bethesda, MD 2013; 19-176.
- French National Agency for Medicine and Health Product Safety/ANSM, Activity Reports, 2009–2021.
- SwissMedic Haemovigilance Annual Reports, 2005–2021.
- Benjamin et al. Transfusion 2017; 57:2946-57.
- Jutzi M. et al. Transfus Med Hemother 2018; 45:151-6.
- AFMPS Hémovigilance Rapport annuel: Belgium. 2016-2018.
- Gammon RR, et al. Transfusion 2022 Mar; 62(3):641-650.
- Lefort, C, et al. Transfusion Clinique et Biologique 2022;29:338.
- Snyder, EL, et al. Transfusion. 2022; 62( 7): 1365–1376.
- McCullough et al: Blood 2004;104(5):1534-1541.
- Snyder E et al. Transfusion 2004;44:1732-1740.
- Corash L et al.Transfusion 2000;40(S10):137.
- Janetzko et al. Transfusion 2005;45:1443-1452.
- Slichter SJ et al. Transfusion 2006; 46(5):731-740.