The Need for Platelet Safety
Platelets are an important therapeutic component for transfusion support of patients with quantitative or qualitative platelet disorders.
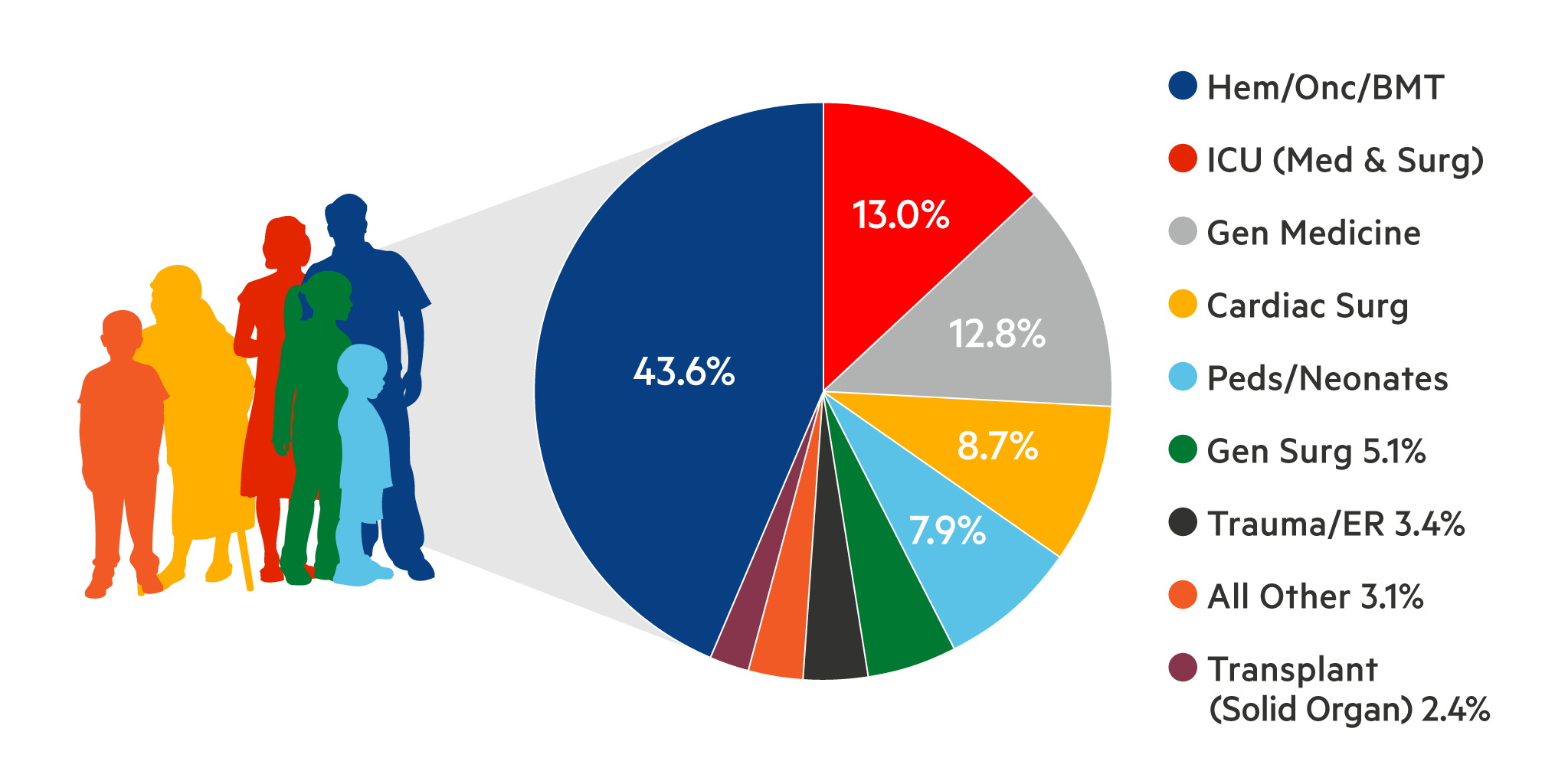
Based on the most recent national survey of blood use, ~2.5 million platelet components were transfused in the US in 20171. Physicians who prescribe platelets, and the patients who receive them, expect their platelets to be safe and effective.
While donor screening and testing have improved, these are inherently reactive measures. They require time to recognize/identify the pathogen, and develop efficacious tests for it. When new pathogens emerge a large proportion of donor populations can be rapidly infected, and a substantial proportion may be asymptomatic3-5. Thus, despite improvements in blood donor testing, there is a need for proactive pathogen reduced platelet products to further reduce the risk of TTI for all indications where platelet transfusion is required, while maintaining therapeutic efficacy and safety.


