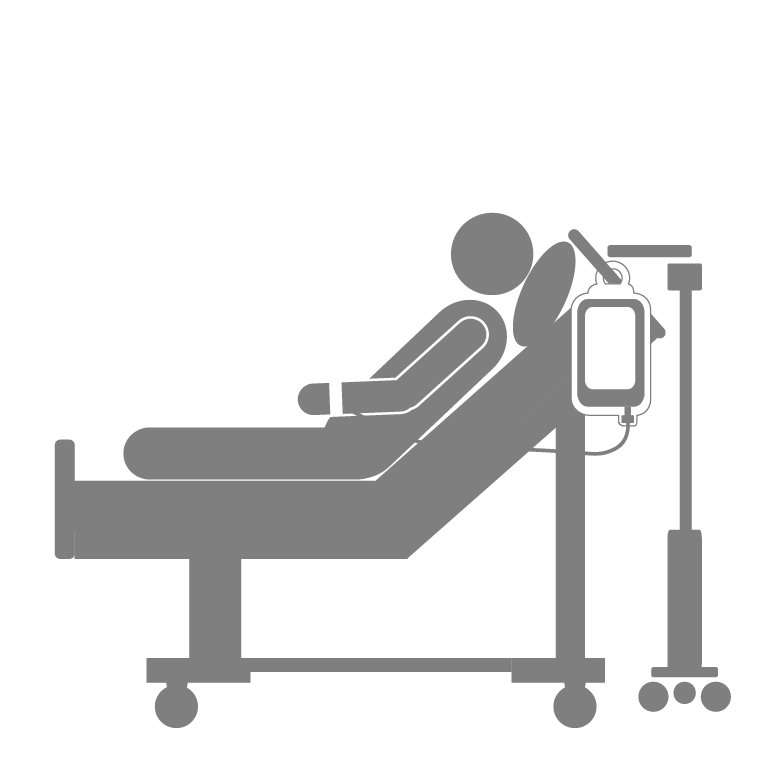
Sepsis and Surveillance
Committed to Blood Safety for Your PatientsFrequency of Contamination
- An estimated 1:1,500 platelet units may contain bacteria1.
- During treatment cycles where six units of platelets are transfused, the risk of a patient receiving a contaminated unit may be ~1:250 to 1:4001,5
- Assuming an active surveillance (vs. passive surveillance) of sepsis occurrence, rates of sepsis risk can be ~1:5,000 units, or about 1:1,000 per patient risk.7
| Bacterial Contamination Risk |
|
 |
~1:1,500 Units to ~1:2,500 Units1,5 |
 |
~1:250 to ~1:400 Patients5,7 |
| Known / Unknown Viral and Parasite Infectious Risk | |
 |
Zika Yellow Fever Babesia |
 |
Chikungunya Dengue Plasmodium |
Transfusion Related Sepsis is Underreported Due To Passive vs. Active Surveillance Methods
- Bacterial contamination of platelets is an ongoing issue…1-6
- …but passive surveillance using diagnostic criteria for septic transfusion reactions rarely identifies these events5
- Over 10 years of active surveillance at a major academic institution, patients continued to receive bacterially-contaminated platelets yet all resulting septic transfusion reactions were missed.5
Active Surveillance: 2007-2013, University Hospitals Case Medical Center5:

51,440 platelet units transfused – all negative by culture
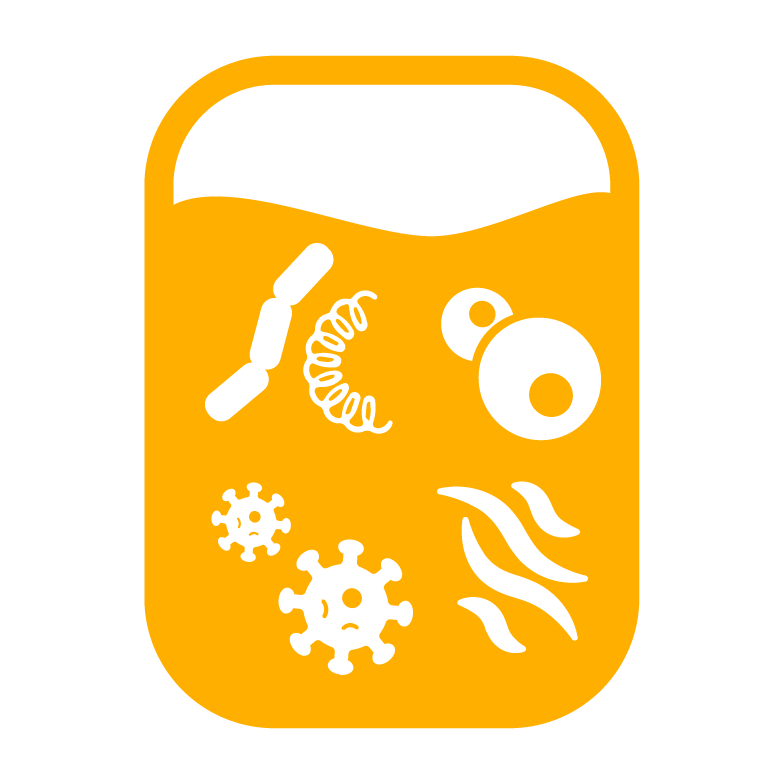
20 out of 51,440 (~1:2,500) transfused platelet units were contaminated with bacteria